Honoring Five Decades of Progress, Commitment, and Support for Liver Patients
(Fairfield, NJ – Jan. 5, 2026) – American Liver Foundation (ALF) proudly announces its 50th anniversary, marking five decades of unwavering commitment to advancing liver health, empowering liver patients and their families, providing groundbreaking public health initiatives and programs, and funding close to $30 million in critical research that supports and educates the 100 million Americans living with some form of liver disease. Since its founding in 1976, ALF has evolved into a leading voice at the forefront of the fight against liver disease, by educating patients and medical professionals through community events, fundraisers and educational webinars and advocating on Capitol Hill for liver health policies. ALF boldly strives to continue forging 50 years forward advancing liver health programs and initiatives that illustrate our continued dedication to liver patients each and every day.
“ALF has achieved numerous milestones that have shaped the landscape of liver health over the past 50 years, and we’re committed to driving progress 50 years forward to educate and support those battling a liver condition and help end liver disease,” said Lorraine Stiehl, Chief Executive Officer of American Liver Foundation. “As we look 50 years forward, we are excited for each milestone that represents 50 years of stepping stones dedicated to improving the lives of liver patients and their families.”
Key highlights through five decades of innovative growth include:
In the 1980s – ALF established a Research Awards Program that has since awarded close to $30 million to 923 scientists; $2.5M of which was awarded in the last two years alone. ALF unveiled a National Toll-Free helpline, 1.800.GO.Liver (1.800.465.4837), that provides trusted liver health information and support in 50 states today and in over 100 languages; and ALF launched Love Your Liver, its first community education program that taught elementary school children about liver health, resisting peer pressure, and saying no to drugs.
In the 1990s – The first Irwin M. Arias Symposium: Bridging Basic Science & Liver Disease launched, uniting global scientists and physicians to bridge basic science and liver disease. Now in its 35th year, this one-day event has featured breakthroughs in diagnosing and treating liver diseases in children and adults worldwide; ALF convened a Scientific Advisory Council of leading hepatitis and liver disease researchers to draft the nation’s first research agenda, setting priorities for Congress to accelerate cures; ALF published the Children’s Liver Research Agenda: A Scientific Blueprint to help guide families coping with pediatric liver disease; and ALF launched Liver Life Walks to bring communities across the country together to raise awareness and funds regarding liver disease in a fun, inspiring way and to provide education and support services to those living with the disease.
In the early 2000s – ALF launched a multi-year, nationwide community education campaign to raise awareness about Hepatitis B (Hep B) called THINK (The Hepatitis Information You Need to Know); ALF’s Board Chair Dr. James Boyer testified before Congress, advocating for increased funding for the National Institutes of Health (NIH), Center for Disease Control’s (CDC) Viral Hepatitis, and Health Resources and Services Administration’s (HRSA) Organ Transplantation; and ALF launched its third national community education program titled FLIP (Fatty Liver Information Program), which laid the groundwork for what would become ALF’s Liver Wellness Program.
In 2010 to 2020 – Music legends Gregg Allman and Natalie Cole joined ALF’s nationwide Hepatitis C virus (HCV) campaign called “Tune in to Hep C”, to raise awareness about HCV; ALF formed its first-ever National Patient Advisory Committee (NPAC) to train liver patients across the nation to use their voices to advocate for change and raise awareness on Capitol Hill about many liver conditions. Today, ALF hosts an annual advocacy day amplifying many voices to fight for liver health policies during our “Liver Life Advocacy Summit.”; ALF launched a live educational series called Ask the Experts, which brings liver health specialists into communities across the country to raise awareness and answer questions about liver health; ALF honored 2020 Nobel Laureates Charles M. Rice, PhD, Harvey Alter, MD, and Michael Houghton, PhD, DSc, at ALF’s 45th Anniversary Leadership Celebration for their discovery of the hepatitis C virus and transformative contributions that led to a cure.
In 2020 to the present – ALF launched Think Liver Think Life, it’s first nationwide public health initiative to screen U.S. adults for metabolic dysfunction-associated steatotic liver disease (MASLD) and liver cancer, raising awareness and connecting communities to the care they need. See if you’re at risk today by taking our free liver health quiz at thinkliverthinklife.org/quiz; ALF created the Bili the Brave toolkit, complete with a plush lion, children’s book, and resources to support children and families affected by biliary atresia; ALF’s Living Donor Network was recently launched to connect non-directed (altruistic) liver donors with transplant centers nationwide to help children and adults in need of a transplant; and ALF launched a Patient Registry to help researchers find better treatments and cures for liver disease.
“ALF expresses profound gratitude to its donors, volunteers, medical partners, scientists and researchers and the liver health community for their steadfast support of our mission for the past 50 years,” said Emmanual Thomas, MD, PhD, FAASLD, ALF Board Chair and Tenured Professor at University of Miami School of Medicine and member of the Sylvester Comprehensive Cancer Center and the Schiff Center for Liver Diseases at University of Miami. “As ALF celebrates its legacy, we look 50 years forward with renewed commitment to fostering hope, advancing innovative breakthroughs, and supporting millions affected by liver disease.”
In honor of our 50th Anniversary milestone, ALF invites all its supporters to join the Liver of Life Society with a monthly donation of $15 or more. By becoming a monthly donor, you can make a lasting difference in the fight against liver disease that will help us sustain vital programs year-round. As a token of our appreciation, all Liver of Life Society members will receive ALF’s commemorative 50th Anniversary tote bag.
“For the past 50 years, ALF has continued to be a beacon of hope for liver patients across the country living with some form of liver disease,” said Dan Weil, immediate past Board Chair of ALF, and spouse to a liver patient for over 25 years. “ALF’s goal is to create a world free from the challenges of liver disease, but we can’t do it without your continued support of our ongoing mission to promote education, advocacy, support services and research for the prevention, treatment and cure of liver disease. Supporting ALF today means you’re helping millions in the future to lead healthier lives and achieve optimal liver health.”
For more information about ALF, go to www.liverfoundation.org and for details regarding the Monthly Donor Campaign, please visit Liver of Life Society – American Liver Foundation. Read ALF’s entire 50th Anniversary timeline. If you have any questions or concerns regarding liver disease, please call our FREE helpline at 1.800.GO.LIVER (800.465.4837).
About the American Liver Foundation
American Liver Foundation (ALF) is a national community of patients, caregivers and medical professionals dedicated to helping people improve their liver health. Providing guidance and life-saving resources, we are a beacon for the 100 million Americans affected by liver disease. We advocate for patients and families, fund medical research and educate the public about liver wellness and disease prevention. We bring people together through our educational programs and events and create a network of support that lasts a lifetime. ALF is the largest organization focused on all liver diseases and the trusted voice for patients and families living with liver disease.
For more information visit:
www.liverfoundation.org
or call: 1 800 GO LIVER (800-465-4837)
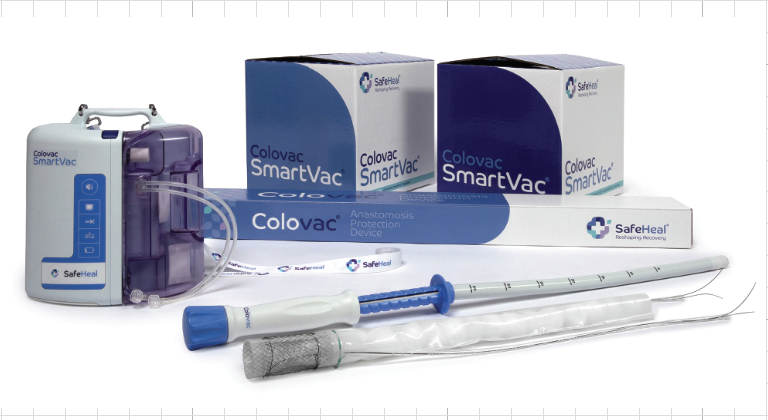
SafeHeal® Announces Successful Launch of SAFE-3CV IDE Study for Colovac® Anastomosis Protection Technology
Breakthrough device promises significantly improved patient recovery after colorectal surgery
PARIS, France/Tampa, FL – SafeHeal®, a leading innovator in the field of colorectal cancer surgery, today announced the first patient enrollment in its pivotal IDE study of Colovac®, a groundbreaking endoluminal bypass sheath. Colovac® is intended as an alternative to a temporary diverting ostomy for patients undergoing colorectal resection. Up to 20 U.S. and European sites will enroll patients in the SAFE-3CV study led by Principal Investigator Patricia Sylla, MD, and EU Principal Investigator Prof. Jérémie Lefevre, which is expected to complete enrollment by late 2026. SAFE-3CV serves as the final phase of a comprehensive study for U.S. market approval and EU post-market surveillance. This two-phase study will enroll up to 252 patients to compare the safety and efficacy of the Colovac® device to the previously collected control data on patients who received the standard-of-care diverting ostomy procedure.
Hôpital Saint-Antoine (AP-HP), Sorbonne Université, Paris, France, under the direction of Prof. Jérémie Lefevre, successfully enrolled the SAFE-3CV study’s first patient. Prof. Lefevre has extensive experience with the Colovac® device as a co-leader of recent studies conducted in the U.S., Europe, and Asia, where Colovac® has demonstrated favorable safety and efficacy as an alternative to ostomy. Based on these prior studies, in August 2025, Colovac® was granted European Union marketing approval under the new Medical Device Regulation (EU MDR 2017/745, Medical Devices, Annex IX Chapter I).
“The current standard-of-care, the use of a diverting stoma, places a significant burden on the patient in terms of associated physical complications, lifestyle compromises, and an extended recovery period,” said Prof. Lefevre. “We welcome Colovac’s potential to offer a less-invasive alternative to a stoma, and we are excited to generate additional evidence behind this innovation.”
As the current standard of care for the surgical treatment of rectal cancer, a diverting ostomy is applied prophylactically to most patients today undergoing a low anterior resection (LAR) and a low anastomosis. The ostomy temporarily diverts the stool away from the healing anastomosis to the outside of the body and into an ostomy bag. In most cases, the ostomy is needed only until the anastomosis has healed, and can then be reversed, typically after 2-6 months. The eventual reversal of the ostomy requires another operation, with a second hospital stay, recovery period and associated complications. In some cases, the ostomy may not be reversed and becomes permanent. In addition to the potential surgical complications associated with ostomy procedures, patients may experience a negative impact on their quality of life due to social isolation, reduced physical activity and/or intimacy issues.
Colovac® is designed to eliminate the need for a temporary stoma in most patients. It aims to improve patient recovery and quality of life by eliminating stoma-related complications, including permanent stoma, and eliminating the physical and emotional burden associated with stoma management and care.

“We are proud to partner with world-class clinicians like Prof. Lefevre and Dr. Sylla to build upon SafeHeal’s already impressive body of evidence, as we drive toward FDA marketing approval of Colovac,” said Chris Richardson, President & CEO of SafeHeal®. “We look forward to making the clinical and economic benefits of this technology available to U.S. patients and providers in the very near future.”
Successful completion of the SAFE-3CV study is the final step in the path to U.S. Food and Drug Administration (FDA) approval and U.S. commercialization of the Colovac® device. The FDA has already granted the product Breakthrough Device designation. Breakthrough Device designation is granted to novel products and provides expedited review of innovative technologies that can improve the lives of people with life-threatening or irreversibly debilitating diseases or conditions.
ABOUT SAFEHEAL®
SafeHeal SAS, headquartered in Paris, France, and its wholly owned U.S. subsidiary, SafeHeal Inc., is a medical device company developing Colovac®, a device intended as an alternative to diverting ostomy in patients undergoing colorectal surgery. Colovac® is a flexible endoluminal bypass sheath designed to reduce the contact of fecal content at the anastomotic site following colorectal surgery. The device is placed endoluminally and remains in place for approximately 10 days, until the body’s natural healing and tissue repair processes are complete, after which it is removed during an endoscopic procedure without the need for a second surgical intervention.
Colovac® enables patients to resume their normal life without the stigma and complications associated with an ostomy procedure. In the U.S., Colovac® is limited by Federal law to investigational use and not currently available for sale.
For more information, please visit:
www.safeheal.com