Introduction
Endoscopic retrograde cholangiopancreaticography (ERCP) is a commonly used therapeutic procedure for diagnostic and therapeutic purposes for various pancreatic-biliary pathologies. Endoscopic sphincterotomy (ES) is a requirement for many biliary interventions. ES can serve as the initial step in the treatment of biliary pathologies, such as the extraction of stones or to allow cholangioscopy or some forms of biliary duct stenting. The outcomes after ES are dependent on the interplay between several factors, including pre-sphincterotomy ductal cannulation, the technique and instrumentation used for sphincterotomy, the post-sphincterotomy therapeutic intervention performed, and finally, the experience and expertise of the endoscopist.1
Approximately 4 to 5% of ES are associated with some degree of adverse event.2 Bleeding is one of the most common adverse events associated with sphincterotomy.3 The bleeding can range from minimal oozing to life-threatening hemorrhage requiring multiple blood transfusions and emergent endoscopic/radiologic/surgical intervention to achieve hemostasis. Understanding the type of sphincterotomy-related bleeding, recognizing high-risk scenarios, and implementing prompt and appropriate hemostatic strategies are key for improving patient outcomes. This article aims to provide a comprehensive overview of the incidence, risk factors, classification, current management strategies, and advanced interventions for refractory cases of endoscopic sphincterotomy-related bleeding.
Incidence
The American Gastroenterological Association (AGA) recognized sphincterotomy as the most important risk factor for bleeding during ERCP as the bleeding from ERCP.3 The incidence of bleeding associated with sphincterotomy can range from 0.5% to 12%.4-8 It is important to note that reporting of the incidence of bleeding varies and is highly dependent on the definition used by the investigators and authors across studies. Some studies define bleeding as a clinical diagnosis (melena or hematemesis) with laboratory evidence of a drop in hemoglobin while others include any bleeding at all, even mild, self-limited oozing. For example, the MESH study by Freeman et al. reported an incidence of bleeding post-ES of 2%.6 Others consider bleeding as endoscopic evidence after performing sphincterotomy. For example, Kim et al. and Leung et al. defined post-sphincterotomy bleeding as an adverse event if the bleeding did not subside after two to three minutes following sphincterotomy.7,8 Hence, the reported incidence was greater, 12.1% and 10.4%, respectively. Nonetheless, the incidence of bleeding as an adverse event post-sphincterotomy has decreased over time. From 10-12% in the 1990s, the current guidelines by the American Society for Gastrointestinal Endoscopy and the AGA cite an expected rate of sphincterotomy-associated bleeding as approximately 1 to 2%, likely representing advanced in sphincterotomy generator waveforms.9
Risk Factors
The risk factors for post-ES bleed include liver cirrhosis, end-stage renal disease, difficult cannulation, precut sphincterotomy and lower ERCP case volumes.9,10,11 The AGA identifies coagulopathy, anticoagulant therapy within three days of procedure, cholangitis, low endoscopist case volume (less than 1 per week), and additional therapeutic maneuvers including ampullectomy as risk factors for bleeding with ES.3 The risks specific for ES can be grouped depending on patient and procedure. A retrospective study by Lin et al. reported significantly increased incidence of post-sphincterotomy bleeding in patients with cirrhosis (OR 3.1), end stage renal disease (OR 3.55), antiplatelet use within three days before or after the procedure (OR 4.95), CBD dilation (OR 1.24) and history of duodenal ulcers (OR 2.06).10 Similarly, the endoscopist experience and the number of sphincterotomies/ERCPs performed also play an important role. For example, mean case volume of ≤ 1/week was associated with 74% significantly higher odds of bleed compared to operators with a high case volume.6
Kim et al. in their prospective analysis found statistically higher bleeding rates with a needle-knife sphincterotome compared to a traditional pull-type sphincterotome (79.4% vs 20.6%, p < 0.025). Moreover, bleeding was significantly more with zipper cuts (3.7% vs 1.2%, p 0.049%).7 However, it is important to note that with recent advancements in the field of advanced endoscopy and newer devices, zipper cuts are extremely rare. Bae et al. showed that the length of ES as an independent risk factor for bleeding.12 Full length (papillary orifice up to the superior margin of the sphincter opening, OR 68.27) was associated with the highest risk followed by medium length (papillary orifice to the midpoint between the proximal hooding fold and the superior margin of sphincter opening, OR 10.97) and then minimal length (papillary orifice to the proximal hooding fold, OR 1). It should be noted that, in general, a complete sphincterotomy is best for the patient. Evidence of extension of previous ES is mixed. While some studies state that it does not affect the risk of bleeding, Leung et al. reported significantly increased risk.3 Prabhu et al. in their review paper explained how sphincterotomy extension was safe without significant risk of adverse events.13
The type of device used for ES also plays an important role. Perini et al. in their study showed that the ValleyLab generator, which is no longer in clinical use, was associated with increased endoscopically evident bleeding (OR 4.02) compared to the microprocessor- controlled generator (ICC 200; ERBE). The ValleyLab generator was associated with increased occurrence of moderate or severe bleeding with increased requirement of urgent endoscopic intervention. It has since been replaced by modern electrosurgical generators.
Classification of Sphincterotomy-Associated Bleeding
Bleeding can be broadly classified as clinically significant or insignificant. Clinically important bleeding can be defined as any bleed that requires intervention (endoscopic hemostasis, transfusion, etc.) and is visible not only through endoscopy but also in the form of melena/hematemesis/hematochezia with a significant drop in hemoglobin.
Cotton et al. proposed a grading system to classify bleeding based on its severity.14 Bleeding can be classified into mild, moderate, and severe. Mild bleeding is defined as clinically apparent bleeding with a hemoglobin drop of less than 3 g/dL that does not require transfusion. Moderate bleeding refers to bleeding that necessitates transfusion of up to four units of blood, without the need for angiographic or surgical intervention. Severe bleeding involves the transfusion of five or more units and/or requires angiographic or surgical management. A clinically insignificant bleed would broadly include all the bleeds that do not fit the above criteria. However, in essence, the distinction between clinically significant and insignificant bleeding after a sphincterotomy is made via clinical judgment and observation and hinges on the impact on the patient’s health and the level of medical intervention required to control the bleeding.
Freeman et al. reported a rate of 2% clinically significant bleeds, out of which 0.6% were mild (not requiring transfusion), 0.9% were moderate (requiring up to 4 units of blood), and 0.5% had severe bleeds (5 or more units of blood, surgery, or angiography).6 Similarly, other studies mostly report mild to moderate bleeding as the most common bleeding severity after sphincterotomy. Leung et al. in their study reported mild, moderate, and severe bleeds as 92.4%, 6.7% and 0.9%, respectively.8
Clinically evident bleeds can become life-threatening emergencies requiring massive blood transfusions, endoscopic interventions, and/or interventional radiology (IR) intervention for embolization. Freeman et al. reported 43.75% post-sphincterotomy patients requiring endoscopic intervention for hemostasis, and 4.2% patients required surgical intervention.6 Death occurred in 4.2% of cases despite aggressive intervention.
Bleeding can also be classified as immediate or delayed based on the timing/onset of bleeding. Immediate bleeding usually occurs during the procedure and is evident by oozing or spurting of blood and is directly observed with the duodenoscope.6,8 However, this may not be clinically significant and in the majority of instances it is self-limiting and managed conservatively.6,8,15
Delayed bleeding refers to a clinically significant bleed occurring after the sphincterotomy, with usually biochemical evidence of hemoglobin drop.8,9 Freeman et al. reported delayed bleeding 1-10 days post-sphincterotomy in 52% cases.6 Lin et al. in their study reported 69.2% immediate and 30.8% delayed bleeds.10 Beyond that, 20% of these severe bleeds were more severe than those with immediate bleeding. Reported delayed bleeding rates were lower in the study by Leung et al., at 4.2% with all cases requiring blood transfusions and repeat endoscopic intervention.8 This indicates that delayed bleeding, although less frequent, can be more fatal compared to immediate bleeding.
Management of Sphincterotomy Induced Bleeding
The cornerstones of managing post-sphincterotomy bleeding are rapid recognition, risk stratification, and immediate availability of appropriate endoscopic tools and expertise. Immediate bleeding, typically identified during ERCP, is addressed using a stepwise endoscopic approach based on bleeding severity and visibility. It should be noted that mild bleeding often stops spontaneously and, if not interfering with visualization, may not require treatment per se.
If treatment is desired, balloon tamponade is usually the first method applied for mild bleeds and this technique can be supplemented with other treatment interventions if adequate hemostasis is not achieved in short order. Injection of epinephrine, hemostatic clips, stents, and thermal coagulation are commonly used endoscopic interventions for hemostasis.4 Topical agents can be used as adjuvants. Delayed bleeding is managed with supportive care and resuscitation with blood products, followed by repeat endoscopy for definitive control if bleeding does not stop spontaneously. In cases with severe bleeding, referral to interventional radiology for angiography with embolization or, rarely, surgical intervention may be necessary. The choice of intervention is often governed by whether the bleed is immediate or delayed, intermittent or ongoing, mild or severe, and the patient’s overall stability. In the following sections, we will discuss the various treatment interventions in detail that can be used to achieve hemostasis in post-sphincterotomy bleeding.
Tamponade
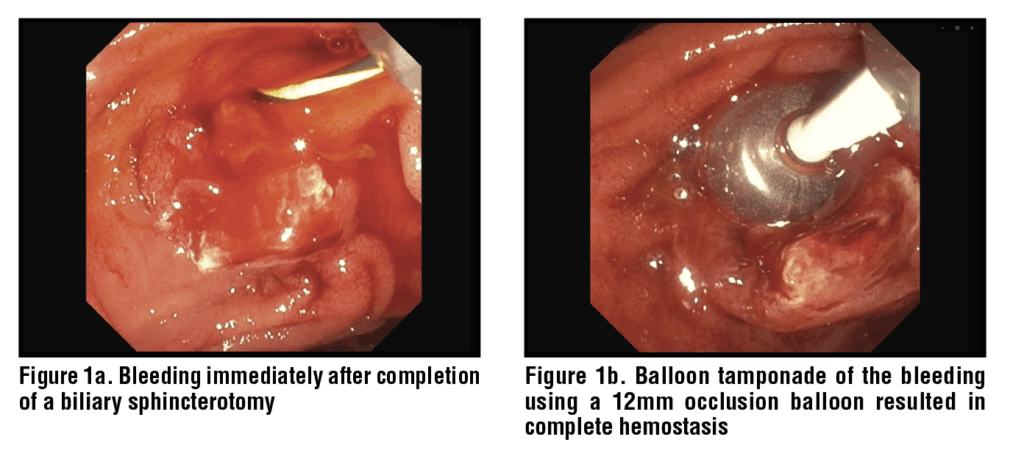
Balloon tamponade is frequently used to control sphincterotomy bleeding and ensure adequate visualization of the bleeding site. (Figure 1) The balloon exerting direct pressure on the bleeding vessel promotes clot formation and hemostasis. This tamponade is most commonly provided using a standard stone extraction balloon or, less frequently, a dilation balloon.16 One advantage of this approach is that the bleeding site can often be directly visualized through the clear plastic of the balloon itself, allowing for interrogation and direct confirmation of ongoing bleeding or cessation of bleeding.
Balloon tamponade is an effective strategy especially for immediate onset bleeding after sphincterotomy. A recent study by Askora et al. showed that balloon tamponade was successful in achieving hemostasis in 10 of 18 subjects (55.6%), and an additional 4 subjects (22.2%) achieved hemostasis after 5 minutes of tamponade.17 Hence, it can be easily used by the endoscopist in cases of immediate post-ES bleed and often the first line of intervention.18 Staritz, et al. used balloon catheters in two cases of severe hemorrhage from the papillary orifice and reported cessation of bleeding after ten minutes.19
Despite the advantages and ease of use of balloon tamponade, it is not free of the risk of adverse events. It can lead to mucosal ischemia through increased pressure application to the mucosal surface during tamponade, although such events are rare. Other adverse events associated with balloon tamponade include bile duct injury, perforation, pancreatitis and cholangitis, and these risks are likely higher with dilation balloons than with retrieval balloons.10,20 Edema or spasm of the pancreatic duct or the biliary duct due to pressure application from the tamponade can contribute to these adverse events. Hence, the endoscopist should be careful in selecting a balloon of appropriate size, ensuring adequate but not undue inflation pressure, and just enough duration of balloon application to avoid adverse events. Despite that, balloon tamponade is a minimally invasive and highly effective intervention for initial use. It is also a cost-effective intervention option compared to more invasive procedures.
Local Injections
Local injection therapy, most commonly with epinephrine, remains a commonly employed technique for controlling post-sphincterotomy bleeding. Injection with diluted epinephrine (1:10000 to 1:20000) is mostly effective in achieving hemostasis by two methods: vasoconstriction and mechanical tamponade by volume of fluid injected into submucosal space surrounding the vessel which compresses it and facilitates thrombosis.15 It should be noted that not all available injection catheters work through a duodenoscope, and simple plastic catheters may be deformed by the elevator mechanism of the duodenoscope and thus fail. Tsou et al. reported epinephrine injection alone was as effective as combination treatment with epinephrine injection and thermotherapy (96.2% vs 100%, p 0.44).22 Apart from epinephrine, hypertonic saline-epinephrine, dextrose-epinephrine, and polidocanol have also been utilized. Sakai et al. reported 100% successful hemostasis with hypertonic-epinephrine injection.23 Hence, local injections can be effectively used as first line agents to achieve hemostasis for mild bleeding and can be used as an adjunctive initial method which can then be followed immediately by a definitive treatment with clipping or thermal coagulation. Recently, fibrin glue has also been reported as an alternative to refractory post-ES bleeding. It contains fibrinogen and thrombin and promotes thrombogenesis to achieve hemostasis. Orlandini et al. reported 91.4% clinical success rate with one injection of fibrin glue in refractory post-ES bleeds.24 Out of the remaining 8.6%, half responded to a second injection of fibrin glue.
Thermal Coagulation
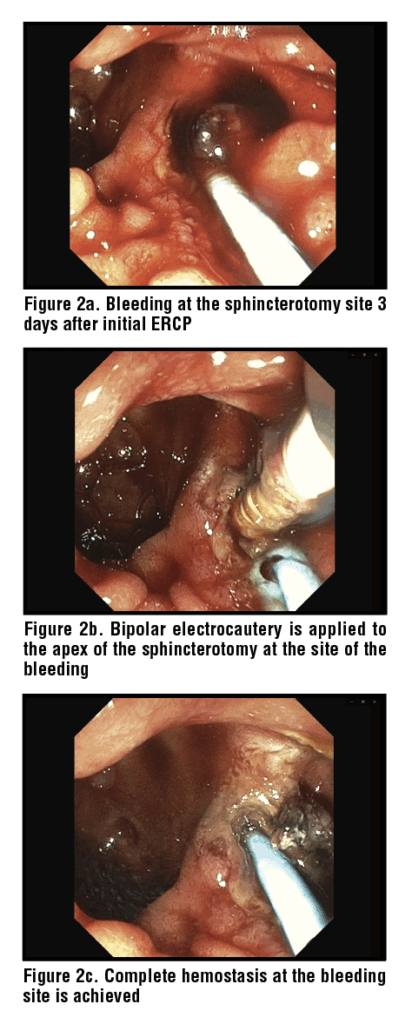
Thermal coagulation plays a significant role in achieving hemostasis when local injection has failed to provide adequate hemostasis. Thermal therapies include monopolar or bipolar electrocautery, heater probes and argon plasma coagulation. (Figure 2) Controlled thermal energy delivered through these techniques cauterizes the bleeding site and can often result in durable hemostasis.25 It should be noted that the cutting wire of the sphincterotome itself can be used to provide monopolar electrocautery to the bleeding site. Katsinelos et al. reported monopolar cautery was 100% successful in controlling post-ES bleeding which was not controlled with epinephrine injection alone.26 Similarly, Sherman et al. reported an 89% hemostasis rate with bipolar cautery in post-ES bleeds.27 A key advantage of thermal methods over injection alone is the creation of a more durable seal with the possibility of coaptation (thus compressing and cauterizing the bleeding vessel at the same time) leading to significantly lower rates of rebleeding. Combination therapies consisting of epinephrine injections and thermal coagulation have also been widely used. Tsou et al. reported 100% success rate in achieving hemostasis across all 37 patients who were treated with combination therapy.22
Clipping
For refractory bleeding not controlled by tamponade or hemostatic topical agents, endoscopic clips can be used. Application of clip is technically challenging with a side viewing endoscope as the small mechanical parts of through-the-scope (TTS) clips can become damaged by the elevator mechanism of the duodenoscope, but some TTS clips work despite this challenge. Cap-assisted end-viewing endoscopes can potentially overcome this problem. Clips can be deployed directly onto the bleeding site, and they can be effective for both active bleeding and for prophylactic prevention. TTS clip application through end-viewing endoscope can achieve successful hemostasis in 90% cases.28 Kim et al. retrospectively evaluated the efficacy of clips for post-ES induced bleeding that was not controlled with epinephrine injection or tamponade. They reported a 100% success rate with no delayed bleeding or complications in all 45 patients treated with clips.29
A propensity score matched analysis conducted by Jinpei et al. in 2024 compared prophylactic hemostatic clip placement after ES with 232 patients in the hemostatic clip group and 161 in the control arm. They reported significantly lower odds of delayed bleeding in the hemostatic clip group arm (OR 0.134, 95% CI 0.025 – 0.719).30 Similarly, Chon et al. reported 100% success rates in all 57 subjects who were managed with endoclip for controlling post-ES bleed.31 Care must be taken to avoid inadvertent closure of the bile or pancreatic duct while placing the clip. However, such adverse events are very rare. Moreover, no significant adverse events have been reported associated with the clips. Clips are a good alternative for refractory post-ES bleeding uncontrolled by injections/ tamponade, which is easier to perform and has low chance of adverse events.
Stenting
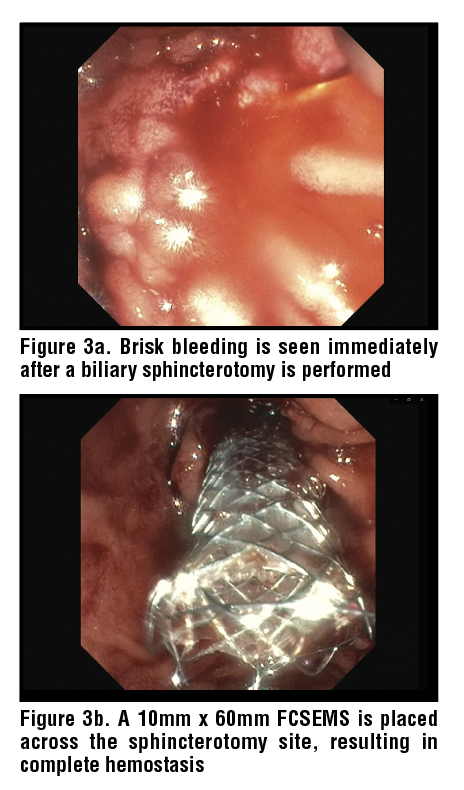
Stents are another treatment alternative for post-ES bleeds uncontrolled with topical agents/ tamponade, and in general covered metal stents are used to treat sphincterotomy bleeding. Itoi et al. suggested 10 mm as an ideal diameter size of the stent.32 Fully covered self-expandable metal stents (FC-SEMS) have been shown to provide excellent tamponade. (Figure 3) Cochrane et al. reported FC-SEMS had significantly lower rate of bleeding at 72 hours compared to traditional endoscopic interventions (tamponade/epinephrine injection).33
In a retrospective study by Bilal et al. including 97 patients, FC-SEMS had a 100% technical success rate in achieving immediate hemostasis and 94% success in achieving durable clinical success for delayed hemostasis. Rebleeding was noted in 6.2% cases which were managed with repeat EGD/ERCP, embolization and surgery.34 The adverse events reported post FC-SEMS included pancreatitis in 4.1% cases and stent migration in 4.1% cases. Even though FC-SEMS have good success rates, due to the higher costs and adverse events associated they are considered as treatment alternatives after conventional endoscopic interventions like tamponate, topical agents or cauterization have failed to control bleeding. FC-SEMS are generally removed several weeks after placement when used to treat sphincterotomy bleeding.
Topical Agents
Topical agents represent a significant advancement in the treatment of GI bleeding, offering a non-mechanical method of hemostasis ideally suited for achieving hemostasis for diffuse hemorrhage or anatomically difficult locations which cannot be controlled by local injections, tamponade or clips. Hemospray (Cook Endoscopy, Winston-Salem NC) acts as a mechanical barrier between the bleeding vessel and the lumen. When applied to the bleeding surface, it absorbs water from the blood and tissue fluids, leading to concentration of clotting factors and platelets. This promotes formation of an adhesive plug that covers the mucosal defect and applies physical tamponade on the bleeding vessel promoting hemostasis.35 Purastat (3-D Matrix, Inc., Tokyo, Japan) is another topical agent used for post-ES bleeds. It is a synthetic hemostatic agent made of amino acids and forms a three-dimensional scaffold after coming in contact with blood.36 This scaffold mimics the human extracellular matrix causing an adhesive effect and promoting hemostasis at the bleeding site. Another agent used is Beriplast (CSL Behring, Marburg, Germany), a fibrin sealant, which mimics final steps of the coagulation cascade to achieve hemostasis.37
Studies have shown high rates of immediate hemostasis (>90%) with Hemospray in achieving hemostasis for gastrointestinal bleeds.38 However, studies evaluating the use of Hemospray for post-ES bleeds are limited. Lesmana et al. in their retrospective study compared Beriplast and Purastat with conventional hemostatic techniques (epinephrine / balloon tamponade) for post-ES bleeds. The study involved 100 patients with 60 patients in the study arm (Beriplast or Purastat) and 40 patients in the control arm (conventional hemostatic agents). They reported a 100% success rate in achieving immediate hemostasis in both the arms. However, two patients (5%) in the control arm had rebleed while none were reported in the study arm. Out of these two patients, one was managed with one out of the two hemostatic agents (Beriplast or Purastat) and the other was managed with argon plasma coagulation.39
A recent RCT was performed comparing the efficacy of a polysaccharide hemostatic powder (XunNing®; Lianbai Bochao Medical Equipment, Chongqing, China) to endoclips for post-ES non-pulsatile bleeding. The study included 104 subjects with 52 each in the study and control arm. Immediate hemostasis was achieved in 100% subjects with polysaccharide hemostatic powder (PHP) while it was 92.3% with endoclip use (p = 0.022). Overall treatment success, which was defined as immediate hemostasis with no delayed bleeding, was significantly more with the PHP use (100% vs 90.4%; P = 0.022). Moreover, hemostasis was achieved in a shorter time with PHP (50.77 vs. 62.81 sec, p = 0.011).40
With topical agents, the primary concern is rebleeding. Hemospray use for gastrointestinal bleeds have shown rebleed rates of as high as 10% to 30%.32 Moosavi et al. reported a case of transient biliary obstruction after application of hemospray for post-ES bleed.41 Despite promising results from Lesmana et al., prospective studies specifically evaluating topical agents for post-sphincterotomy bleeding are needed.39
IR/surgery for Profound Post-ES bleeding
With advancements in the endoscopic techniques, only a small subset of patients with post-sphincterotomy bleeding will require intervention beyond endoscopy. IR-guided embolization is the preferred next-step modality for hemodynamically unstable patients with ongoing bleeding that is refractory to endoscopic control or when endoscopic visualization is impossible. The IR approach involves angiographic localization of bleeding source, typically the posterior pancreaticoduodenal artery and/or one of the branches of the gastroduodenal artery followed by embolization with coils, particles or glue. Maleux et al. reported 97% successful embolization in post-ES bleeding that was refractory to medical and endoscopic treatment.42 If bleeding is from duodenal varices, IR approaches may have difficulty in fully stopping it. Recurrent bleeding occurred in 9% cases and 30-day mortality was 20.6%. The high mortality rates in this study were attributed to hemostatic disorders characterized by increased international normalized ratio (INR) and activated partial thromboplastin time (aPTT) with statistically significant correlation between the 30-day mortality and elevated levels of INR and aPTT (P value of 0.008 and 0.012, respectively).
Shenbagaraj et al., in their retrospective study, reported 100% success (n=4) with embolization in post-ES bleeds that were refractory to endoscopic intervention.43
Surgery remains the definitive treatment of last resort, reserved for cases who have failed embolization or when bleeding is too massive for endoscopic or angiographic control. The surgical options include open surgical vessel ligation or surgical repair of the duodenum and papilla. Most commonly performed surgery is duodenotomy with direct suture ligation of bleeding vessels at the sphincterotomy site.4 More extensive procedures such as pancreaticoduodenectomy are rarely required and carry significant morbidity and mortality.
The choice between IR guided intervention and surgery is multidisciplinary, dependent on patient clinical stability, anatomy and expertise available at the treatment center. However, the minimally invasive nature of angioembolization is considered as a bridge between failed endoscopy and high-risk surgery.
Conclusion
Post-ES bleeding is a well- reported adverse event which, in general, requires a structured approach for management. The cornerstone of treatment is endoscopic intervention. Epinephrine injection, balloon tamponade, thermal coagulation, and the use of endoscopic clips are foundational treatment modalities. For refractory cases, FC-SEMS and topical hemostatic agents offer valuable alternatives before considering angioembolization or surgery.
References
References
1. Neuhaus H. Biliary sphincterotomy. InERCP 2019 Jan 1 (pp. 137-147). Elsevier.
2. Perini RF, Sadurski R, Cotton PB, Patel RS, Hawes RH, Cunningham JT. Post-sphincterotomy bleeding after the introduction of microprocessor-controlled electrosurgery: does the new technology make the difference?. Gastrointestinal endoscopy. 2005 Jan 1;61(1):53-7.
3. Adler DG, Lieb JG, Cohen J, Pike IM, Park WG, Rizk MK, Sawhney MS, Scheiman JM, Shaheen NJ, Sherman S, Wani S. Quality indicators for ERCP. Official journal of the American College of Gastroenterology| ACG. 2015 Jan 1;110(1):91-101.
4. Ferreira LE, Baron TH. Post-sphincterotomy bleeding: who, what, when, and how. Official journal of the American College of Gastroenterology| ACG. 2007 Dec 1;102(12):2850-8.
5. Goodall RJ. Bleeding after endoscopic sphincterotomy. Annals of the Royal College of Surgeons of England. 1985 Mar;67(2):87.
6. Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ, Lande JD. Complications of endoscopic biliary sphincterotomy. New England Journal of Medicine. 1996 Sep 26;335(13):909-19.
7. Kim HJ, Kim MH, Kim DI, Lee HJ, Myung SJ, Yoo KS, Park ET, Lim BC, Seo DW, Lee SK, Min YI. Endoscopic hemostasis in sphincterotomy-induced hemorrhage: its efficacy and safety. Endoscopy. 1999 Aug;31(06):431-6.
8. Leung JW, Chan FK, Sung JJ, Chung SS. Endoscopic sphincterotomy-induced hemorrhage: a study of risk factors and the role of epinephrine injection. Gastrointestinal endoscopy. 1995 Dec 1;42(6):550-4.
9. Chandrasekhara V, Khashab MA, Muthusamy VR, Acosta RD, Agrawal D, Bruining DH, Eloubeidi MA, Fanelli RD, Faulx AL, Gurudu SR, Kothari S. Adverse events associated with ERCP. Gastrointestinal endoscopy. 2017 Jan 1;85(1):32-47.
10. Lin WC, Lin HH, Hung CY, Shih SC, Chu CH. Clinical endoscopic management and outcome of post-endoscopic sphincterotomy bleeding. PloS one. 2017 May 17;12(5):e0177449.
11. Nelson DB, Freeman ML. Major hemorrhage from endoscopic sphincterotomy: risk factor analysis. Journal of clinical gastroenterology. 1994 Dec 1;19(4):283-7.
12. Bae SS, Lee DW, Han J, Kim HG. Risk factor of bleeding after endoscopic sphincterotomy in average risk patients. Surgical endoscopy. 2019 Oct 15;33(10):3334-40.
13. Parbhu S, Adler DG. Extension of a Prior Biliary or Pancreatic Sphincterotomy: Efficacy, Outcomes, and Adverse Events. PRACTICAL GASTROENTEROLOGY. 2016 Jan 1;40(1):21-32.
14. Cotton PB, Lehman G, Vennes J, Geenen JE, Russell RC, Meyers WC, Liguory C, Nickl N. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointestinal endoscopy. 1991 May 1;37(3):383-93.
15. Wilcox CM, Canakis J, Mönkemüller KE, Bondora AW, Geels W. Patterns of bleeding after endoscopic sphincterotomy, the subsequent risk of bleeding, and the role of epinephrine injection. Official journal of the American College of Gastroenterology| ACG. 2004 Feb 1;99(2):244-8.
16. Rustagi T, Jamidar PA. Endoscopic retrograde cholangiopancreatography–related adverse events: general overview. Gastrointestinal Endoscopy Clinics. 2015 Jan 1;25(1):97-106.
17. Askora AA, Ibrahim AM, Elgohary M, Saad N, Abu Taleb MA. Efficacy and Safety of Balloon Tamponade in Control of Post Sphincterotomy Bleeding During Endoscopic Retrograde Cholangiopancreatography. Zagazig University Medical Journal. 2025 May 1;31(5):1911-27.
18. Ghoz HM, Dayyeh BK. Hemorrhagic complications following endoscopic retrograde cholangiopancreatography. Techniques in Gastrointestinal Endoscopy. 2014 Oct 1;16(4):175-82.
19. Staritz M, Ewe K, Goerg K, Meyer zum Büschenfelde KH. Endoscopic balloon tamponade for conservative management of severe hemorrhage following endoscopic sphincterotomy. Zeitschrift fur Gastroenterologie. 1984 Nov 1;22(11):644-6.
20. Mustafa FM, Ali HF. Endoscopic management of ERCP bleeding. The Egyptian Journal of Hospital Medicine. 2019 Apr 1;75(5):2888-93.
21. Matsushita M, Uchida K, Okazaki K. Effective injection site on endoscopic injection therapy for postsphincterotomy bleeding: apex or oral?. Official journal of the American College of Gastroenterology| ACG. 2008 Jun 1;103(6):1569-70.
22. Tsou YK, Lin CH, Liu NJ, Tang JH, Sung KF, Cheng CL, Lee CS. Treating delayed endoscopic sphincterotomy-induced bleeding: epinephrine injection with or without thermotherapy. World Journal of Gastroenterology: WJG. 2009 Oct 14;15(38):4823.
23. Sakai Y, Tsuyuguchi T, Sugiyama H, Nishikawa T, Kurosawa J, Saito M, Tawada K, Mikata R, Tada M, Ishihara T, Yokosuka O. Hypertonic saline-epinephrine local injection therapy for post-endoscopic sphincterotomy bleeding: removal of blood clots using pure ethanol local injection. Surgical Laparoscopy Endoscopy & Percutaneous Techniques. 2013 Aug 1;23(4):e156-9.
24. Orlandini B, Schepis T, Tringali A, Familiari P, Boškoski I, Borrelli de Andreis F, Perri V, Costamagna G. Fibrin glue injection: Rescue treatment for refractory post-sphincterotomy and post-papillectomy bleedings. Digestive Endoscopy. 2021 Jul;33(5):815-21.
25. Kuran S, Parlak E, Oguz D, Cicek B, Disibeyaz S, Sahin B. Endoscopic sphincterotomy–induced hemorrhage: treatment with heat probe. Gastrointestinal endoscopy. 2006 Mar 1;63(3):506-11.
26. Katsinelos P, Kountouras J, Chatzimavroudis G, Zavos C, Fasoulas K, Katsinelos T, Pilpilidis I, Paroutoglou G. Endoscopic hemostasis using monopolar coagulation for postendoscopic sphincterotomy bleeding refractory to injection treatment. Surgical Laparoscopy Endoscopy & Percutaneous Techniques. 2010 Apr 1;20(2):84-8.
27. Sherman S, Hawes RH, Nisi R, Lehman GA. Endoscopic sphincterotomy-induced hemorrhage: treatment with multipolar electrocoagulation. Gastrointestinal endoscopy. 1992 Mar 1;38(2):123-6.
28. Liu F, Wang GY, Li ZS. Cap-assisted hemoclip application with forward-viewing endoscope for hemorrhage induced by endoscopic sphincterotomy: a prospective case series study. BMC gastroenterology. 2015 Oct 15;15(1):135.
29. Kim TH, Sohn YW. Mo1428 Hemoclip Application Using CAP-Fitted Forward Endoscopy to Treat Post-Sphincterotomy Bleeding in Patients Undergoing ERCP. Gastrointestinal Endoscopy. 2015 May 1;81(5):AB416.
30. Dong J, Feng Q, Teng G, Niu H, Bian D. Application of a New Hemostatic Clip to Prevent Delayed Bleeding After Endoscopic Sphincterotomy: A Propensity Score–matched Analysis. Journal of Clinical Gastroenterology. 2024 Jul 1;58(6):614-8.
31. Chon HK, Kim TH. Endoclip therapy of post-sphincterotomy bleeding using a transparent cap-fitted forward-viewing gastroscope. Surgical Endoscopy. 2017 Jul;31(7):2783-8.
32. Itoi T, Yasuda I, Doi S, Mukai T, Kurihara T, Sofuni A. Endoscopic hemostasis using covered metallic stent placement for uncontrolled post-endoscopic sphincterotomy bleeding. Endoscopy. 2011 Apr;43(04):369-72.
33. Cochrane J, Schlepp G. Comparing endoscopic intervention against fully covered self-expanding metal stent placement for post-endoscopic sphincterotomy bleed (CEASE Study). Endoscopy International Open. 2016 Dec;4(12):E1261-4.
34. Bilal M, Chandnani M, McDonald NM, Miller CS, Saperia J, Wadhwa V, Singh S, Cohen JM, Berzin TM, Sawhney MS, Pleskow DK. Use of fully covered self-expanding metal biliary stents for managing endoscopic biliary sphincterotomy related bleeding. Endoscopy International Open. 2021 May;9(05):E667-73.
35. Holster IL, van Beusekom HM, Kuipers EJ, Leebeek FW, de Maat MP, Tjwa ET. Effects of a hemostatic powder hemospray on coagulation and clot formation. Endoscopy. 2015 Jul;47(07):638-45.
36. Subramaniam S, Kandiah K, Thayalasekaran S, Longcroft-Wheaton G, Bhandari P. Haemostasis and prevention of bleeding related to ER: The role of a novel self-assembling peptide. United European gastroenterology journal. 2019 Feb;7(1):155-62.
37. Eberhard U, Broder M, Witzke G. Stability of Beriplast® P fibrin sealant: Storage and reconstitution. International journal of pharmaceutics. 2006 Apr 26;313(1-2):1-4.
38. Chahal D, Sidhu H, Zhao B, Jogendran M, Dahiya M, Tandon P, Donnellan F. Efficacy of Hemospray (TC-325) in the treatment of gastrointestinal bleeding: an updated systematic review and meta-analysis. Journal of Clinical Gastroenterology. 2021 Jul 1;55(6):492-8.
39. Lesmana CR, Sandra S, Paramitha MS, Gani RA, Lesmana LA. Endoscopic Management Using Novel Haemostatic Agents for Immediate Bleeding during Endoscopic Retrograde Cholangio-Pancreatography. Canadian Journal of Gastroenterology and Hepatology. 2023;2023(1):5212580.
40. Li H, Zuo J, Wang W, Wu S, Zhao Y, Wei Y, Song J, Zhang Z, Yao W, Wang J, Liu C. Efficacy of Polysaccharide Hemostatic Powder on blood oozing among patients with Post-Endoscopic Sphincterotomy Bleeding: A Randomized Controlled Trial. Official journal of the American College of Gastroenterology| ACG. 2022 May 12:10-4309.
41. Moosavi S, Chen YI, Barkun AN. TC-325 application leading to transient obstruction of a post-sphincterotomy biliary orifice. Endoscopy. 2013 Dec;45(S 02):E130-.
42. Maleux G, Bielen J, Laenen A, Heye S, Vaninbroukx J, Laleman W, Verhamme P, Wilmer A, Van Steenbergen W. Embolization of post-biliary sphincterotomy bleeding refractory to medical and endoscopic therapy: technical results, clinical efficacy and predictors of outcome. European radiology. 2014 Nov;24(11):2779-86.
43. Shenbagaraj L, White J, Czajkowski M, Allison M. PTU-116 Delayed post sphincterotomy bleeding and management–4 year single centre experience.
 Archit Garg
Archit Garg Douglas G. Adler
Douglas G. Adler